Part 5 of a series on rethinking science and technology for the 21st century
Last time in this series of occasional blogs, I made the rather bold statement that while science and technology are going to have a highly visible impact on our lives over the next few decades, progress is going to be underpinned in most cases by our increasing control over materials at the invisible nanoscale. It isn’t exactly intuitive why this should be the case though—how on earth can engineering matter on a scale a billion time smaller than the average person be so important?
In trying to answer this question, I want to take a rather unconventional approach and explore three advantages of working at this scale: Smallness, strangeness and sophistication.
 Smallness. Size matters—it’s something we all understand intuitively. There are occasions when you can do something with a small object or device that would be impossible otherwise. This photo from Ilan Kelman for instance illustrates the idea perfectly: There are times that “smallness” gets you to places that larger objects can’t reach—like parking spaces!
Smallness. Size matters—it’s something we all understand intuitively. There are occasions when you can do something with a small object or device that would be impossible otherwise. This photo from Ilan Kelman for instance illustrates the idea perfectly: There are times that “smallness” gets you to places that larger objects can’t reach—like parking spaces!
It’s easy to see how making things that we can see and touch small can enhance their value. But the utility of smallness doesn’t stop when things become invisible to the naked eye. All the way down to the nanometer scale, there are opportunities to make things work better or work differently by making them small.
Here’s a very trivial example of smallness making a difference at the nanometer scale, but it’s a useful illustration of why size matters:
Silver is a great antimicrobial agent. It’s been used for millennia to prevent infections from spreading and is one of the reasons why “silverware” is—or used to be—made of the metal.
But it’s not that easy to use. Large lumps of metal aren’t always that easy to incorporate into products that you want to keep sterile or have antimicrobial properties.
One solution is convert the individual silver atoms into charged ions that can be dissolved in liquids and incorporated into other substances. As its the ionic form of silver that is most harmful to microbes, this makes a lot of sense. But ionic silver isn’t that easy to use either. Say you have a silk scarf or a wound dressing you want to imbue with antimicrobial properties. Getting those silver ions in there without changing the physical feel and nature of the material is a tough challenge.
 This is where smallness comes in. Make the silver metal into nanometer-sized particles, and it becomes relatively easy to get it into a wide range of products. Because these are particles we are dealing with, there isn’t so much complex chemistry behind using them. And because they are so small, they don’t unduly affect the feel and performance of the products they are used in. As an added advantage, replacing a few large particles with millions of small ones increases the chances of microbes coming into contact with them manyfold.
This is where smallness comes in. Make the silver metal into nanometer-sized particles, and it becomes relatively easy to get it into a wide range of products. Because these are particles we are dealing with, there isn’t so much complex chemistry behind using them. And because they are so small, they don’t unduly affect the feel and performance of the products they are used in. As an added advantage, replacing a few large particles with millions of small ones increases the chances of microbes coming into contact with them manyfold.
Because of the advantages of smallness when it comes to using silver as an antimicrobial, there has been an explosion of products using silver nanoparticles—everything from refrigerators to socks to toothpaste. And all because smallness gets you to new places.
It’s a trivial example, but it does illustrate an important way in which “smallness” through increased control over matter at the nanoscale leads to added value.
It’s not the only way though—there is also strangeness.
Strangeness. No two questions about it, things can get a little weird down at the nanoscale. This is good – it means that controlling matter at this scale opens up a whole new toolbox of material properties that can be put to good use.
 Vicki Colvin at Rice University came up with a great analogy for strangeness a few years back. It went something like this: Imagine you have a cat. It looks like a cat, sounds like a cat, smells like a cat. Now, imagine you have a technology that allows you to make that cat smaller. As you shrink your cat down, it gets smaller and smaller, but still retains its essential cat-ness. But imagine reaching a point where suddenly, instead of looking, smelling, sounding like a cat, your cat becomes a dog!
Vicki Colvin at Rice University came up with a great analogy for strangeness a few years back. It went something like this: Imagine you have a cat. It looks like a cat, sounds like a cat, smells like a cat. Now, imagine you have a technology that allows you to make that cat smaller. As you shrink your cat down, it gets smaller and smaller, but still retains its essential cat-ness. But imagine reaching a point where suddenly, instead of looking, smelling, sounding like a cat, your cat becomes a dog!
This is the very essence of strangeness—materials behaving in unexpected and sometimes radically different ways when they are engineered at a nanometer scale. This doesn’t always happen—it depends on the material and the scale on which the material is being engineered—but in some cases the changes in behavior can be startling.
A good example is found in the metal gold.
Gold is an inert, yellowish metal—everyone knows this. It’s lack of reactivity is why so much jewelry is made from the stuff (it doesn’t tarnish), and in part why it holds its value. But form gold into particles just a new nanometers across, and everything changes—the metal does the equivalent of transforming from a cat into a dog. Instead of appearing yellowish in color, the particles now appear red, and become highly chemically active.
 This change in color has been exploited for millennia in glass-making (unbeknownst to the glass-makers, who had no idea they were making and using nanoparticles), with perhaps the most famous example being the Lycurgus cup from Roman times. Illuminated from behind, the gold nanoparticle-containing dichroic glass that the cup is made from appears deep red in color.
This change in color has been exploited for millennia in glass-making (unbeknownst to the glass-makers, who had no idea they were making and using nanoparticles), with perhaps the most famous example being the Lycurgus cup from Roman times. Illuminated from behind, the gold nanoparticle-containing dichroic glass that the cup is made from appears deep red in color.
This strange behavior has a lot to do with how the movement of electrons in materials is affected when they are engineered at a nanometer scale. As these movements affect everything from electrical conductivity and interactions with electromagnetic radiation—including visible light—to how a material conducts heat, nanometer-scale engineering allows scientists and engineers to tap into material properties that are rarely accessible without control at this level.
But it’s not enough to have a smorgasbord of strangeness at out fingertips—we also need the ability to use these unusual properties. And this is where sophistication comes in.
Sophistication. As humans, we are pre-programmed to build things. As kids, we start early—usually with large blocks. But we soon learn that there are limits to what can be made with these rather awkward building blocks, and so we progress on to finer blocks—think of it as graduating from wooden blocks to Duplo. However, it isn’t long before we outgrow these bricks and crave something smaller with which to create increasingly sophisticated structures. And so we discover that ultimate building medium—Lego.
It’s a rather tongue in cheek analogy, but it illustrates something we all know: The smaller the building blocks we use, the more sophisticated the products we can make. This applies at the human scale, but it just as equally applies at the nanometer scale. In fact, being able to build with nanometer-scale clumps of atoms and molecules gives us perhaps what is the ultimate construction set. And before anyone interjects with “surely that’s just chemistry,” the distinction here is the ability to put these small clumps where we want them with nanometer scale precision. This is sophistication at the nanometer scale, and opens up new possibilities in engineering materials and products with enhanced or unique properties.
It’s probably fair to say that we are just beginning to scratch the surface of what can be achieved through sophisticated nanometer-scale engineering, but already there are examples that hint at the potential that is opening up.
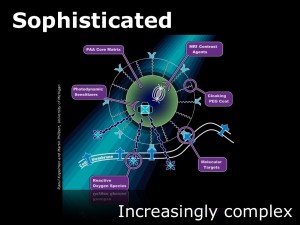 Here you see a schematic of an actual nanometer-scale particle developed by Raoul Kopelman and Martin Philbert at the University of Michigan. What is particularly interesting is the sophisticated way this particle has been engineered at the nanoscale to carry out a number of tasks.
Here you see a schematic of an actual nanometer-scale particle developed by Raoul Kopelman and Martin Philbert at the University of Michigan. What is particularly interesting is the sophisticated way this particle has been engineered at the nanoscale to carry out a number of tasks.
The core particle is coated with a thin layer of PolyEthylene Glycol (PEG) to make it invisible to the body’s defense systems. It is also covered with molecules that enable it to attach to a specific target cell—a particular cancer cell in this case. Internally, the nanoparticle has been engineered with a contrast-enhancing agent, meaning that when sufficient particles are attached to the tumor being treated, they can be seen using imaging techniques like MRI.
Then the really clever bit—the particles have been engineered with a sensitizer. In essence, this is a component that causes the particle to do something when it receives a signal. In this case, when the particle is illuminated with a particular wavelength of light, it releases chemicals to kill the cancer cell it is attached to.
This “smart” particle represents an incredible degree of sophistication at the nanometer scale, and does what it does—destroys cancer cells without affecting healthy cells—because of this sophistication. And it’s only one example from an increasing number of applications that demonstrate what can be achieved when we have the sophistication to build things at close to the scale of individual atoms and molecules.
At the end of the day, smallness, strangeness and sophistication don’t tell you everything you need to know to understand why an increasing ability to control matter at the nanoscale is so important. But they do provide a pretty good insight—dare I say, a sophisticated insight—into what can be achieved by working at this scale.
They also create a bridge between two largely separate spheres that is poised to take our control over the world in which we live to an entirely new level. But more of that next time.
Notes
Rethinking science and technology for the 21st century is a series of blogs drawing on a recent lecture given at the James Martin School in Oxford. This is a bit of an experiment—the serialization of a lecture, and a prelude to a more formal academic paper. But hopefully it will be both interesting and useful. I’ll be posting a “rethinking science and technology” blog every week or so, interspersed with the usual eclectic mix of stuff you’ve come to expect from 2020science.
Previously: Control: Gaining mastery over the world at the finest level

Hi Andrew! You’ve touched on something I’ve been struggling with i.e. explaining to people about nanotechnology and its potential impact. I’ve done a few presentations for social science and/or artsy audiences and talk with friends about it. Most of them enter into a coma-like state when I go on about the size. That billionth of a metre thing is a killer and I’m still searching for good metaphors. I’ll add the cat and dog thing to my cache of metaphors.
Hi Maryse,
You blew my cover – I was attempting to write about the ideas surrounding nanotechnology without using the term “nanotechnology” – which comes with rather a lot of baggage 🙂
This is a tough thing to explain, in part because everything is happening at a scale smaller our brains are programmed to deal with, and in part because the reasons why we are doing this tend to be a little woolly.
And on the scale thing – I’m not yet convinced that trying to explain how small the nanoscale is is that useful (although I’m as guilty as the next person in having my favorite scale analogies).
In particular, the thing about a nanometer being 100,000 times smaller than a human hair underwhelms me – it never seems that big a deal! Human hairs are pretty fat, all things considered – I can see individual hairs on someone’s head from a few feet away, so they are not that small. And something that is 100,000 times smaller than something I can easily see with my naked eyes doesn’t sound that impressive!
But I may be wrong about this – if anyone out there is impressed by the idea of something that is 100,000 times smaller than one of your hairs on your head (assuming you have any), let me know.
I am desperately trying to come up with other analogies for the nanoscale that are accurate. Sometimes I just say the actual 1 billionth of a meter. Or sometimes I use more vague terms like 1 million could dance on the head of a pin, or the width of just a couple atoms, or as wide as the DNA helix (but no one understands how small that is). As a writer, explaining nanoscale every single day drives me nuts!!! The NNI diagram helps: http://www.nano.gov/html/facts/The_scale_of_things.html
The 2006 National Geographic description isn’t too bad:
http://ngm.nationalgeographic.com/2006/06/nanotechnology/kahn-text/2
I’ve been known to compare the difference between the human scale and the nanoscale to the difference in size of the moon and Twinkie -but it only works for some audiences, and even then gets tired rather fast!
http://www.penmedia.org/video/maynard.html
And as @myerberlow pointed out on Twitter, George Whitesides approach to explaining the nanoscale is helpful – although I disagree with him that a human hair is at the limits of what can be resolved with the naked eye:
http://tinyurl.com/dnchd9
Andrew:
That’s a fascinating laymen’s-terms description of nanotechnology.
As for the human hair-to-nanometer analogy, I don’t think it is as much a “what the naked eye can resolve” comparison as much as a ready reference everyone is familiar with.
I work with particulate matter, typically a few nanometers to a few micrometers (gigantic for you!) The EPA regulations talk about PM2.5, and the most common analogy is – 1/40th the diameter of a human hair.
I tried to tweet back take a look at
http://tinyurl.com/dnchd9
Myer
R Subramanian – think you are right: The human hair thing is about familiarity, not size as such. In this case, it works well as a way of comparing the unfamiliar with the familiar. But as a way of “wow-ing” people, I’m still not convinced!
Matter smaller by 100,000 times is not more than just comparing how small is that. It might be the simplest way to understand the size and dimension of the matter. But analogies for the nanoscale with more accurate ways could not be avoided. Anyhow thanks for excellent information and useful links.
A gentle correction and a request…
The Lycurgus Cup uses DICHROIC glass, not dichloric glass. As a warm glass artist, I use dichroic glass in a lot of my work.
I discovered your (excellent, BTW) blog while looking for images of the Lycurgus Cup. Could you possibly tell me the source of the image you have used? I would like to get permission to use that image in my website and brochure when explaining dichroic glass and its history.
Thanks,
Carol
Hi Carol,
Many thanks for the correction (which has been made) – embarrassed that it appeared in the first place!
The images were taken from the internet and placed on the same slide – I’ll dig up the source and send it to you via email.