A new paper published on-line today in Nature Nanotechnology hints that some nanoparticles could cause damage to cells on the other side of normally tight barriers – such as the blood brain barrier or the placenta – without actually crossing the barriers. It’s a study that could raise concerns over the safe medical use of nanoparticles, at a time when the first human trials of “smart nanoparticle” therapeutics are being discussed.
Using an artificial system designed to investigate cellular barriers, Gevdeep Bhaba and co-authors show that high concentrations of Cobalt-Chromium alloy nanoparticles on one side of a tightly meshed layer of cells can cause measurable DNA damage to cells on the other side. And they seem to do this without actually crossing the cellular barrier.
I’m not sure how much attention this paper will get, but given its apparent relevance to harm occurring across the placental barrier, there could be some pickup beyond the usual scientific outlets. And interestingly, it is being published at the same time as the first human trials for a “smart nanoparticle” based cancer therapy are being reported – that’s a juxtaposition that could drive a substantial amount of interest in the research.
As I was asked to comment on it prior to its release, I thought it worth jotting some notes down here on the work, just in case anyone’s interested (I’ll be in the thick of a workshop on emerging technologies and emerging economies when the paper is published on-line, so this post is being written some time ahead of it going live).
In brief, the paper (Nanoparticles can cause DNA damage across a cellular barrier, by Gevdeep Bhaba et al., Nature Nanotechnology. DOI: 10.1038/NNANO.2009.313) describes a set of experiments carried out using an artificially grown layer of cells on a porous support. The cells (BeWo cells for the interested, derived from a human trophoblast choriocarcinoma cell line) were grown as a multi-layered barrier, to simulate tight barriers in the body like the placental barrier. On one side of this layer of cells were placed human fibroblast cells. On the other side, Cobalt-Chromium alloy particles (CoCr particles) were placed. Following introduction of the particles, the fibroblasts were checked for DNA damage using an alkaline comet assay.
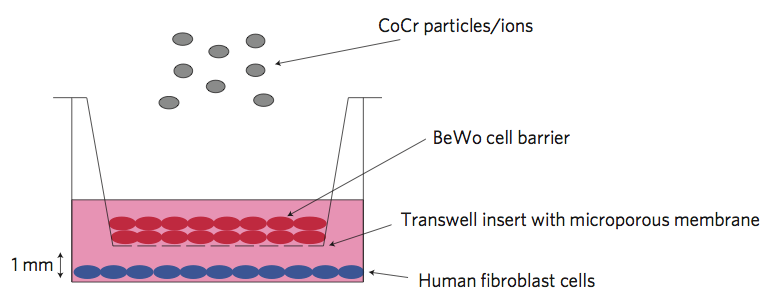
Schematic of the system used by Bhabra and colleagues to investigate the potential for CoCr particles to cause DNA damage across tight cellular barriers (Nature Nanotechnology, DOI: 10.1038/NNANO.2009.313)
As you would expect in a good study, DNA damage was measured under a number of conditions, to identify what was going on. Nanometer-scale and larger CoCr particles were used to see whether size was important. Cobalt and Chromium ions were also used, to see whether the presence of dissolved metals was significant. Particles were also introduced directly to the fibroblasts, to see what happened in the absence of the cellular barrier. In addition, the concentration of Cobalt and Chromium was measured below the cellular barrier to see how much stuff (if any) got through. And the barrier cells were treated with agents designed to block different transmission routes for certain substances, to get a handle on whether DNA damage was being caused by stuff penetrating through the barrier, or being generated (and subsequently released) from within the barrier.
The upshot of all this was that the researchers found evidence that placing Cobalt or Chromium one one side of the barrier caused measurable DNA damage in the fibroblasts on the other side, and that the damage seemed to be associated with chemicals generated within the cellular barrier by the metals. In other words, the combination of CoCr particles and cellular barrier seemed to lead to DNA damage the other side of the barrier, even though the particles didn’t cross it!
The authors of the paper conclude:
We suggest that an evaluation of nanoparticle safety should not rely on whether they fail to gain access to privileged sites. Instead there should also be an evaluation of their genotoxic potential for both direct and indirect effects to avoid any potential risks to targets on the distal [far] side of cellular barriers.
However, while this is an interesting paper, it wold be dangerous to speculate too far on what its relevance to nanoparticle safety. When asked to comment briefly on the paper by the Science Media Center in the UK, this is what I wrote:
This is a study that raises an intriguing question – can foreign materials in the body cause harm across barriers like the placenta and the blood-brain barrier without actually crossing the barriers? Evidence is presented that suggests there is some possibility of this occurring. But the results should be treated with a high degree of caution until more is known. In particular:
The effects seen are do not seem to be confined to nanoparticles alone. There is some evidence that even large particles containing Cobalt and Chromium – the two specific materials studied here – can exert their influence across barriers in the body.
No evidence is presented to suggest that this is a way in which all nanoparticles can cause harm, as opposed to the specific types of nanoparticles tested.
From these results, it is not possible to say whether the observed effects could occur under real-life conditions, or whether harm could be caused at realistic exposure levels. The concentrations of material used were very high – the equivalent of the placenta in a 9 months pregnant woman being exposed to approximately 4 – 40 grams of material. Whether such high exposures to materials like the ones used will ever occur is questionable.
While the study opens up new avenues of research, and should be of particular interest to scientists developing new nanoparticle-based drugs and medical devices, it is too early to say whether materials in the body – including nanomaterials – are likely to cause damage across normally tight barriers like the placenta.
In other words, a fascinating piece of science that raises the possibility of a novel way in which materials could cause harm, but which sheds little light on the likelihood of this being a significant concern from real products in real people.
The bottom line here is that, while this is a scientifically interesting study, it is far removed from implying that specific types of nanoparticles in the body could actually cause significant harm in this way. Certainly, it suggests more research is needed in this area – especially as an increasing number of drugs and medical devices are developed that rely on nanoparticles, and as these products enter the human trials phase. But at the moment, the data do not support nanoparticle-related DNA damage across the placenta (or any other tight biological barrier) as being a probable cause of serious harm.

This is interesting I was commenting on the dangers of the use of aluminum in vaccines to a colleague who is a physician and I came across some studies that would support your findings. It has been shown that the ionic charge of the metals can cause damage to the outer cell wall of neuroblastoma cells. It appears the presence of the charged disrupts the K+ and ionic channels in the cell outer membrane. The study also showed the presence of the aluminum disrupts the cell inner integrity. The metal doesn’t have to cross cell cellular barrier to cause damage.
I would suggest that the ionic nature of nanomettalic particles be more thoroughly studied before being used in the field and commercialized. I also suggest that the previous study be conducted using nanomettalic particles that are bound and don’t have a charge. I bet you will find little if any cell disruption or DNA damage. I would hope this approach allows for the use of metallic nanoparticles in the treatment for diseases.
Just someone Who Cares!
Anthony Zelinko
Thanks Anthony,
I think the good news here is that the more we know about how materials like nanoparticles and metal ions interact with biological systems, the better positioned we are to use these interactions for good, and avoid inadvertently causing harm.
I love the way you explain things.
How strange that the quantities used in this study should have been so large. It’s as if the researchers were going on a premise similar to ‘our bodies absorb Vitamin D from sunshine and we need Vitamin D, so let’s see what happens when we give people 24 hours a day of sunshine rather than just the 10-20 minutes one needs to get one’s recommended daily dose.’
Thanks Ruth.
Something that is reasonably common in research like this is that you use large quantities of material to give you a measurable effect. It’s an approach that can provide insight into biological mechanisms, but the results are often hard to relate to real-world conditions without further research.
I can’t access the full article. What was the effect of Co or Cr ions (as opposed to metallic nanoparticles)?
Apologies for the slow response Marty. Eyeballing the data (fig 1 b & c in the paper), introducing either 0.04 µM or 0.4 µM Cr6+ or 0.4 µM Co2+ ions led to a response in the Comet assay that was around half that of either the nano-sized or micrometer-sized CoCr particles (concentrations 0.036 mg/cm2 and 0.36 mg/cm2). However, the response was still significant, compared to the control.
There was surprisingly little difference in response between the nano- and micro- particles, or between the two different doses (whether using small or large particles, or ions) – which I find worrying from an experimental design perspective.
Dear Andrew,
thanks for yet another good review. I like the way you reflect critically on science, it is really inspiring.
In my case, this article made me think that very often those studies (as you have commented above) use very high concentrations of NPs/chemicals to get a measurable effect. Althought this might obvious for a scientist, the “public” struggles in understanding the role of dose (and exposure) in those studies. I dealt in the past with NGOs and in their talks they gave for granted that toxicological studies use concentrations of NPs to which people would be likely to be exposed to. This si a big knowledge gap! What can we do to fill the gap? Is it totally unrealistic to ask that reseachers doing these experiment to always do a “real-life” control experiment with a more realistic dose? Maybe more a comment then a question…
Luisa
I think this is a big knowledge gap – between scientists, as well as scientists and others – although I’m not sure how best to fill it.
I think there are probably a couple of issues here – understanding why something is done in the way that it is, and understanding the dangers of mis-framing a piece of research. For example, a study using high doses might offer insight into specific modes of action within biological systems, while not being designed to shed light on toxicity. If the study is either evaluated as a toxicity study, or promoted as such, there is a great deal of room for misunderstanding.
How you deal with this, beyond making it very clear what the context of the research was, I’m not sure.